WHY
BIMZELX®?
BIMZELX WORKS TO REDUCE,
RELIEVE, AND HELP CONTROL HS SYMPTOMS
HALF OF PEOPLE SAW REDUCED HS SYMPTOMS
In two clinical studies, 48% and 52% of people saw at least a 50% reduction in the number of inflamed nodules and abscesses, with no increase in abscesses or draining tunnels, at 16 weeks (vs 29% and 32% taking placebo).*
75% REDUCTION IN HS SYMPTOMS
In two clinical studies, 33% and 36% of people saw at least a 75% reduction in the number of inflamed nodules and abscesses, with no increase in abscesses or draining tunnels, at 16 weeks (vs 18% and 16% taking placebo).*
Maintained improvement up to 1 year
In a separate analysis, nearly 3 out of 5 people saw at least a 50% reduction of HS symptoms at week 16 and of those 89% of people maintained it for up to one year. Because these results were from a less rigorous analysis, no conclusions can be drawn.†
50% reduction at week 2
In two clinical studies, 22% and 20% of people saw at least a 50% reduction in the number of inflamed nodules and abscesses, with no increase in abscesses or draining tunnels, at week 2 (vs 14% and 8% taking placebo). This timepoint was not the main focus of the studies.*
*Results are from a 320-mg dose of BIMZELX, given as an injection under the skin every 2 weeks for 16 weeks. Results may vary. Everyone responds to treatment differently.
†Results are from a 320-mg dose of BIMZELX, given as an injection under the skin every 2 weeks for 16 weeks followed by every 4 weeks. Results may vary.
Everyone responds to treatment differently.
*Results are from a 320-mg dose of BIMZELX, given as an injection under the skin every 2 weeks for 16 weeks. Results may vary. Everyone responds to treatment differently.
†Results are from a 320-mg dose of BIMZELX, given as an injection under the skin every 2 weeks for 16 weeks followed by every 4 weeks. Results may vary.
Everyone responds to treatment differently.
BIMZELX works to treat
HS in a different way
HS symptoms you see and feel, like abscesses, are due to an overactive immune system producing too many inflammatory proteins.
BIMZELX is the only FDA-approved medication that targets two of these key proteins, IL-17A and IL-17F, blocking their ability to contribute to inflammation.
BIMZELX BLOCKS BOTH IL-17A and IL-17F
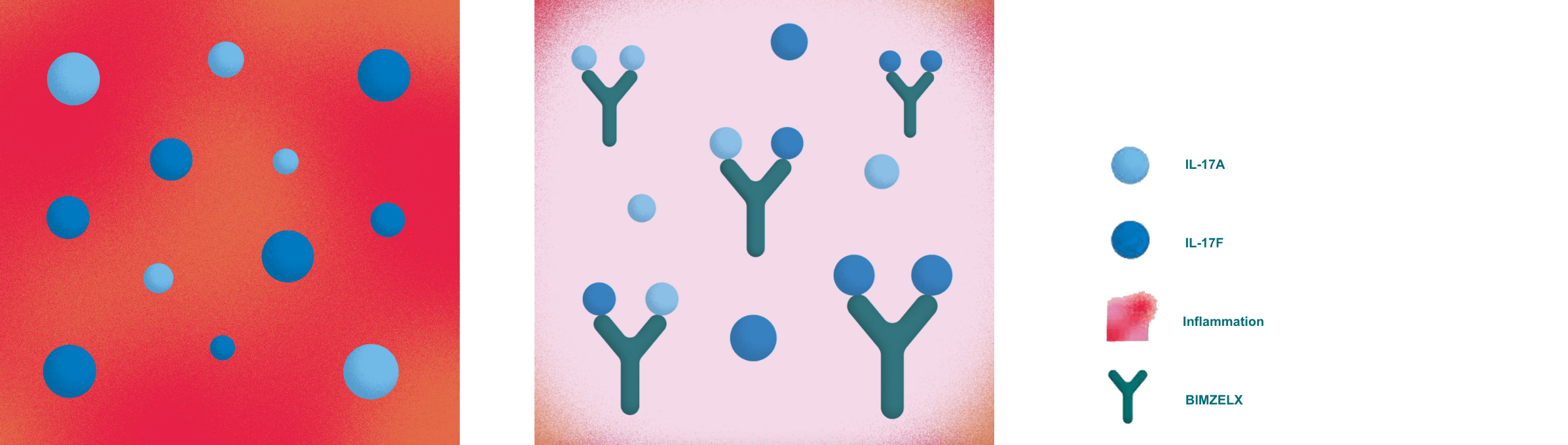
For illustrative purposes only. Based on preclinical data.
Talk to your dermatologist about BIMZELX and your treatment goals.
Find out more about treating HS with BIMZELX.

*For eligible, commercially insured patients only. View complete eligibility requirements and terms at www.bimzelx.com/patient-support/navigate-benefits.
†Nurse Navigators do not provide medical advice and will refer you to your healthcare professional for any treatment-related questions.
†Nurse Navigators do not provide medical advice and will refer you to your healthcare professional for any treatment-related questions.
IMPORTANT SAFETY INFORMATION:
BIMZELX is a medicine that affects your immune system and may increase your risk of serious side effects, including suicidal thoughts and behavior, serious infections including tuberculosis, liver problems, and inflammatory bowel disease.
BIMZELX® is a prescription medicine used to treat adults with moderate-to-severe hidradenitis suppurativa.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about BIMZELX® (bimekizumab-bkzx)?
BIMZELX is a medicine that affects your immune system. BIMZELX may increase your risk of having serious side effects, including:
- Suicidal thoughts and behavior have happened in some people treated with BIMZELX. Get medical help right away or call the National Suicide and Crisis Lifeline at 988 if you, your caregiver or your family member notice in you any of the following symptoms:
- new or worsening depression or anxiety
- thoughts of suicide, dying, or hurting yourself
- changes in behavior or mood
- acting on dangerous impulses
- attempt to commit suicide
- Infections. BIMZELX is a medicine that may lower the ability of your immune system to fight infections and may increase your risk of infections, including serious infections.
- Your healthcare provider should check you for infections and tuberculosis (TB) before starting treatment with BIMZELX.
- If your healthcare provider feels you are at risk for TB, you may be treated with medicine for TB before you begin treatment with BIMZELX and during your treatment.
- Your doctor should watch you closely for signs and symptoms of TB during and after treatment with BIMZELX. Do not take BIMZELX if you have an active TB infection.
Before starting BIMZELX, tell your healthcare provider if you:
- are being treated for an infection
- have an infection that does not go away or that keeps coming back
- have TB or have been in close contact with someone with TB
- think you have an infection or have symptoms of an infection such as:
- fever, sweats, or chills
- muscle aches
- cough
- shortness of breath
- blood in your phlegm
- weight loss
- warm, red, or painful skin or sores on your body different from your psoriasis
- diarrhea or stomach pain
- burning when you urinate or urinating more often than normal
After starting BIMZELX, call your healthcare provider right away if you have any of the signs of infection listed above. Do not use BIMZELX if you have any signs of infection unless you are instructed to by your healthcare provider. See “What are the possible side effects of BIMZELX?” for more information about side effects.
What is BIMZELX?
BIMZELX is a prescription medicine used to treat:
- adults with moderate-to-severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or treatment using ultraviolet light alone or with pills (phototherapy)
- adults with active psoriatic arthritis
- adults with active non-radiographic axial spondyloarthritis with objective signs of inflammation
- adults with active ankylosing spondylitis
- adults with moderate-to-severe hidradenitis suppurativa.
It is not known if BIMZELX is safe and effective in children.
Before using BIMZELX, tell your healthcare provider about all of your medical conditions, including if you:
- have any of the conditions or symptoms listed in the section “What is the most important information I should know about BIMZELX?”
- have a history of depression, or suicidal thoughts or behavior
- have liver problems
- have inflammatory bowel disease (Crohn’s disease or ulcerative colitis)
- have recently received or are scheduled to receive an immunization (vaccine). You should avoid receiving live vaccines during treatment with BIMZELX.
- are pregnant or plan to become pregnant. It is not known if BIMZELX can harm your unborn baby.
- If you become pregnant while taking BIMZELX, you are encouraged to enroll in the Pregnancy Registry, which is used to collect information about the health of you and your baby. Talk to your healthcare provider or call 1-877-311-8972 to enroll in this registry or visit http://mothertobaby.org/pregnancy-studies/.
- are breastfeeding or plan to breastfeed. It is not known if BIMZELX passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with BIMZELX.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the possible side effects of BIMZELX?
BIMZELX may cause serious side effects. See “What is the most important information I should know about BIMZELX?”
- Elevated liver enzyme levels. Your healthcare provider will do blood tests to check your liver enzyme levels before starting treatment and during treatment with BIMZELX. Your healthcare provider may temporarily stop or permanently stop your treatment with BIMZELX if you develop liver problems. Call your healthcare provider right away if you develop any signs or symptoms of liver problems, including:
- pain on the right side of your stomach-area
- feeling very tired
- loss of appetite
- nausea and vomiting
- itching
- dark urine
- light-colored stool
- yellowing of your skin or the whites of your eyes
- Inflammatory bowel disease. New cases of inflammatory bowel disease or “flare-ups” have happened with BIMZELX. If you have inflammatory bowel disease (Crohn’s disease or ulcerative colitis), tell your healthcare provider if you have worsening disease symptoms during treatment with BIMZELX or develop new or worsening signs of Crohn’s disease or ulcerative colitis.
The most common side effects of BIMZELX in people treated for psoriasis and moderate-to-severe hidradenitis suppurativa include: upper respiratory tract infections, headache, herpes simplex infections (cold sores in or around the mouth), small red bumps on your skin, feeling tired, fungal infections (oral thrush or infection in the mouth, throat, skin, nails, feet, or genitals), pain, redness or swelling at injection site, stomach flu (gastroenteritis), and acne.
The most common side effects of BIMZELX in people treated for psoriatic arthritis include: upper respiratory tract infections, headache, urinary tract infections, oral thrush or infections in the mouth, and diarrhea.
The most common side effects of BIMZELX in people treated for non-radiographic axial spondyloarthritis include: upper respiratory tract infections, headache, cough, joint pain, tonsillitis, urinary tract infections, oral thrush or infections in the mouth, diarrhea, feeling tired, muscle aches, and an increase in liver enzyme levels.
The most common side effects of BIMZELX in people treated for ankylosing spondylitis include: upper respiratory tract infections, headache, pain at injection site, vaginal yeast infections, oral thrush or infections in the mouth, diarrhea, and rash.
These are not all of the possible side effects of BIMZELX. Call your doctor for medical advice about side effects.
Use BIMZELX exactly as your doctor tells you to use it.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-10881-800-FDA-1088.
Thank you!
We appreciate your response.
US-BK-2400082
